Data science is playing a pivotal role in redefining the life science businesses starting from discovery phases to commercial activities. Diverse data sources, humongous volume and varied data types such as text, audio, images and video, demand adoption of numerous available algorithms and models for data processing and analysis to aid decision making.
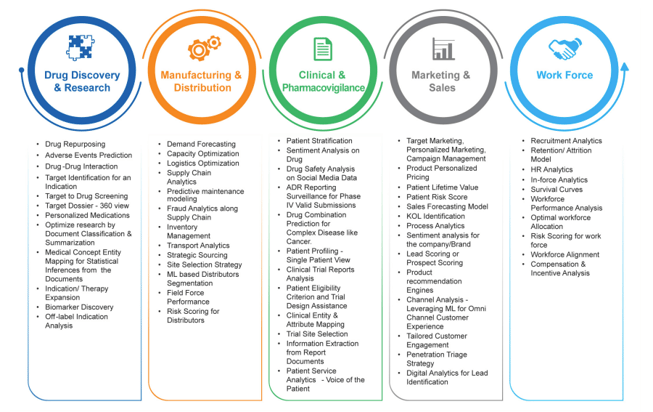
The use cases illustrated below are few examples where benefits of Data Science tools and techniques can be leveraged in healthcare industry.
Data science brings innovative solutions for every stage in the business life cycle. Repertoire of data and modeling techniques provide opportunity to fasten the drug discovery, literature mining, adverse events prediction and even personalized medications using EHR and genomics data. Manufacturing and distribution can be benefitted from use cases like predictive maintenance, demand forecasting, and fraud analytics in supply chain etc. Clinical and pharmacovigilance studies can be supported using sentiment analysis, patient stratification, information extraction and analysis from ADR systems etc. Marketing and sales activities can also use tailored marketing, finding key opinion leaders, cohort analysis etc. For workforce analytics, attrition analysis, regional and personal risk scoring, life time prediction can aid value to a healthcare firm.
Details pertaining to few of these use-cases:
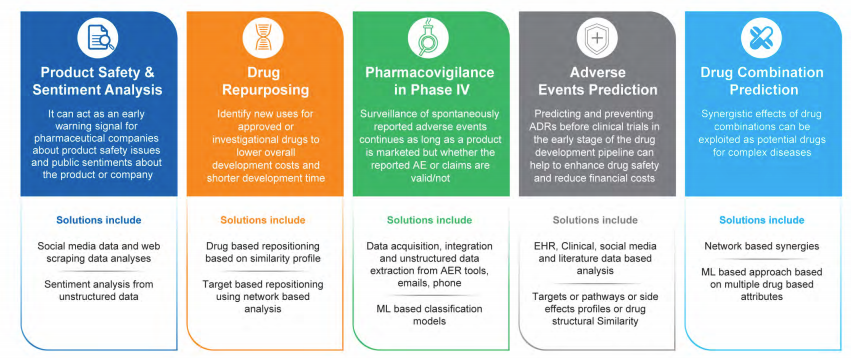
- Adverse Events Prediction: Predicting and preventing ADRs before clinical trials in the early stage of the drug development pipeline can help to enhance drug safety and reduce financial costs. Analysis of EHR, clinical, social media feeds and literature data along with targets or pathways or side effects profiles or structural details from various resources could create a data science related comprehensive analysis and an effective pipeline for AE prediction.
- Product Safety or Sentiment Analysis: It can act as an early warning signal for pharmaceutical companies about product safety issues and public sentiments about the product or company. ML models can be constructed to analyzed historical as well as live streaming social media feeds and web scraping data for sentiment analysis.
- Pharmacovigilance in Phase IV or Post – Marketing Survey: Surveillance of spontaneously reported adverse events continues as long as a product is marketed but whether the reported AE or claims are valid/not remains questionable. Machine Learning based classification models can offer a solution that include data acquisition, integration and unstructured data extraction from AER tools, emails, telephonic conversation related text data.
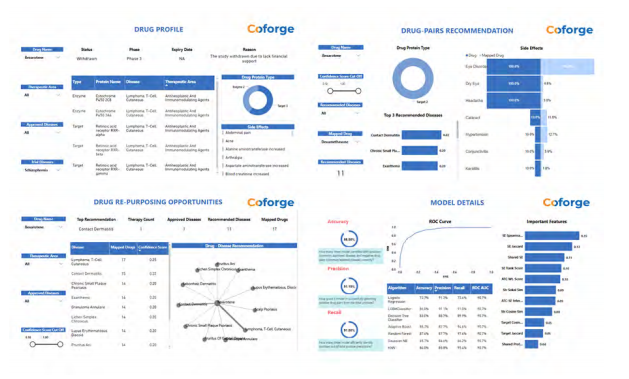
Drug Repurposing
Drug repositioning of failed or existing drugs near patent expiration can provide other tremendous benefits like >90% reduction in development cost, 50% reduction in time and 1000 times better success rate. Most drugs failed in clinical studies due to lack of efficacy or unexpected toxicities. Reasons attributed to inadequate understanding of drug action due to complexity of human disease biology. Identifying new diseases for existing failed or expired drugs needs a system biology approach.
Our solution offers automated data ingestion and integration using drug identifiers from pharma, partners, CRO, third-party and authenticated open data sources. Phenotypic, therapeutic, structural, and genomic details
measures. Our machine learning pipeline offers easy integration with client’s proprietary and external data with dynamic selector for best algorithms. It generates a comprehensive drug-drug interaction network overlaid to drug-disease network to compute confidence score for the mapped diseases. Readily available dashboards provide quick exploration of drug descriptors, most similar drug and top recommended diseases network.