The challenges faced by Life Sciences have always been unique and more so in the recent years. Disruptions due to the pandemic is driving the industry into unchartered territories resulting in new opportunities but the hurdle of regulations are tighter than ever. The traditional, tried and tested, methods are giving way to new processes demanding flexibility, remote working, and cloud efficiency. Coforge, along with our partner Veeva, has been working towards addressing these challenges and have a robust suite of solutions in place for a holistic transformation. Our collaboration is designed to alleviate the pain points of commercial, clinical, and regulatory aspects of drug development.
Coforge Advantage
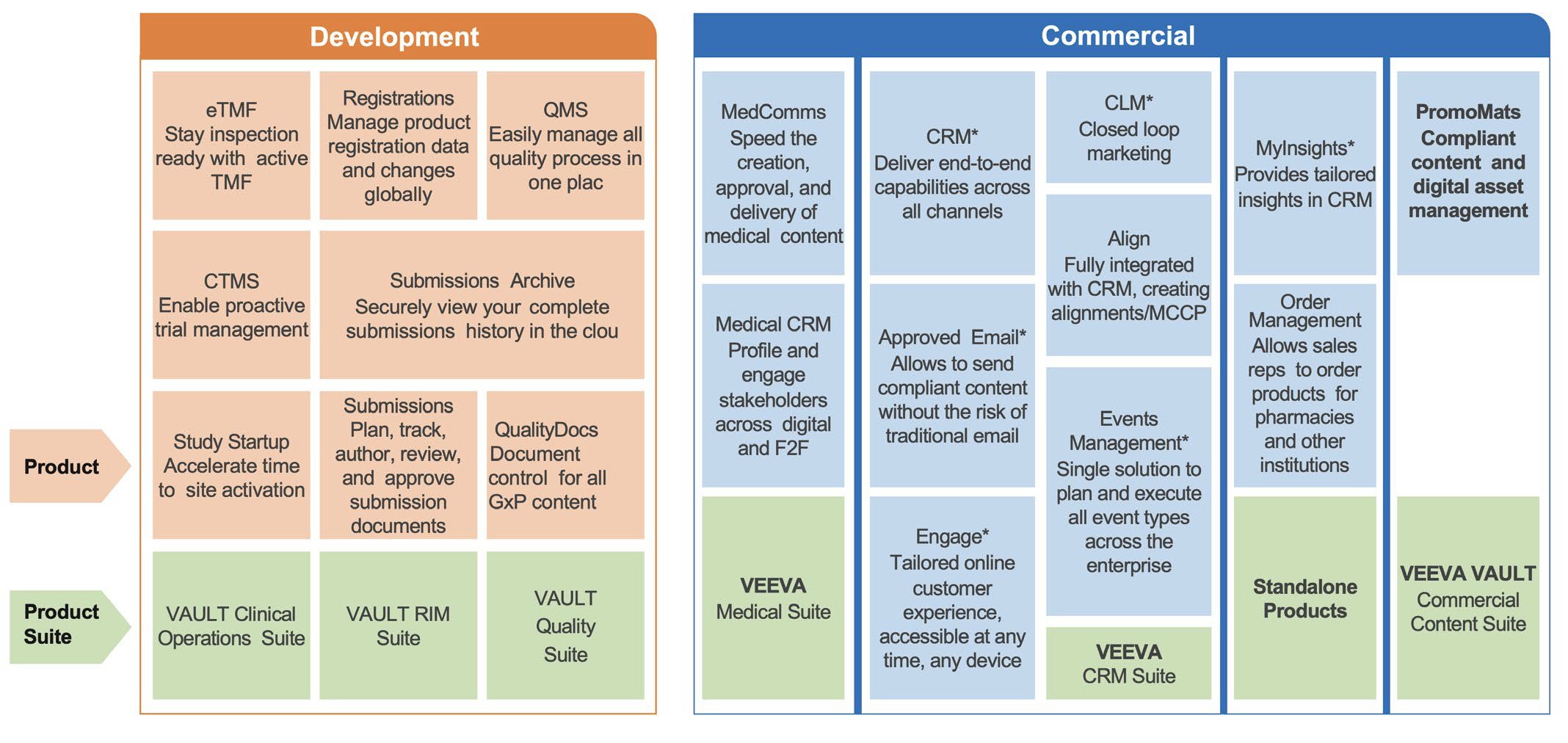
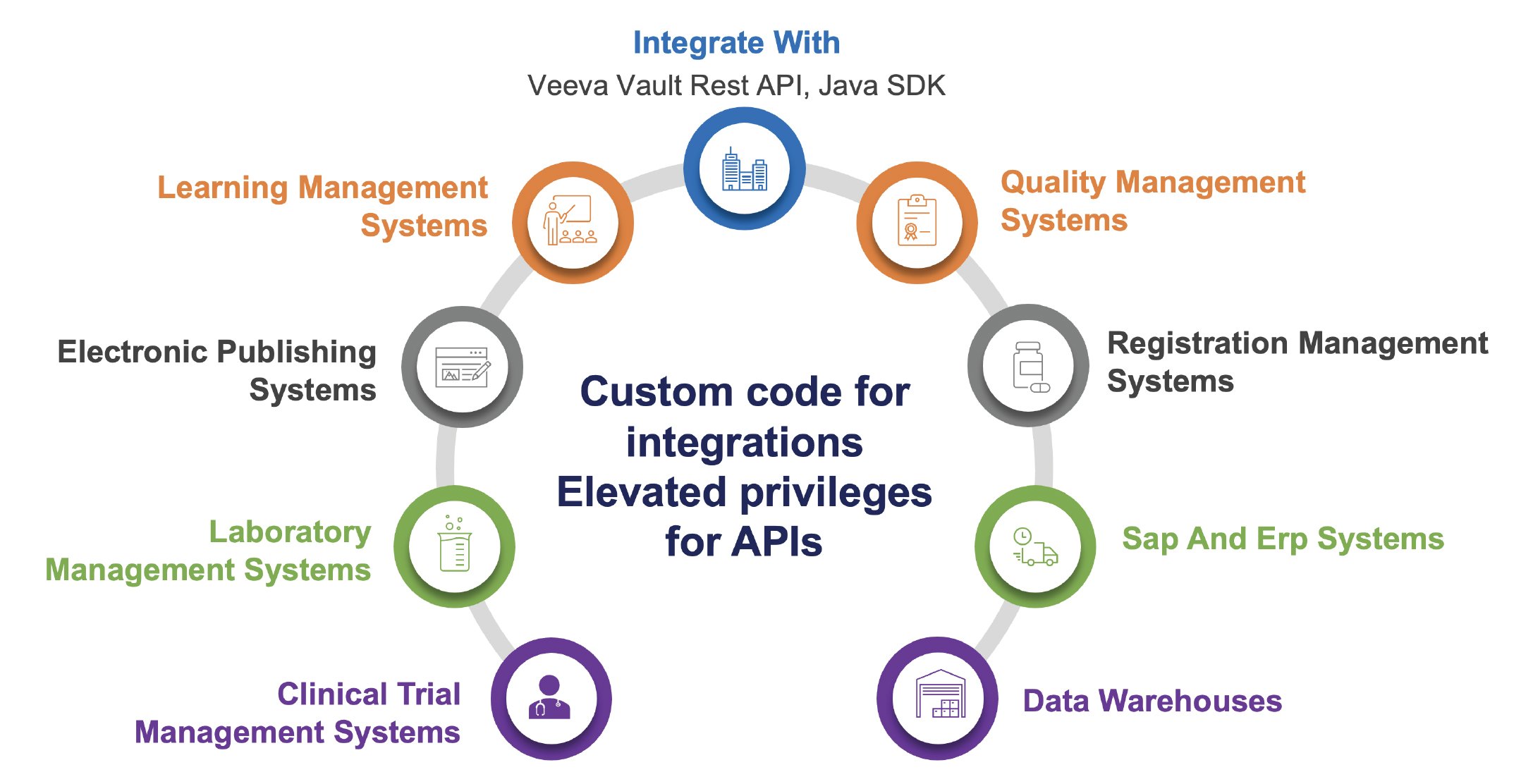
Coforge, with our deep industry knowledge and core expertise in Veeva, can execute a customised transformation strategy aligned with your organisation’s business goals. Our team of experts, rich in technical and domain knowledge, would seamlessly integrate the numerous systems with Veeva for a cohesive ecosystem. Our proven capabilities in Veeva, combined with the rich experience enables in bringing advanced solutions and approaches that offer significant value.
Regulatory Compliance
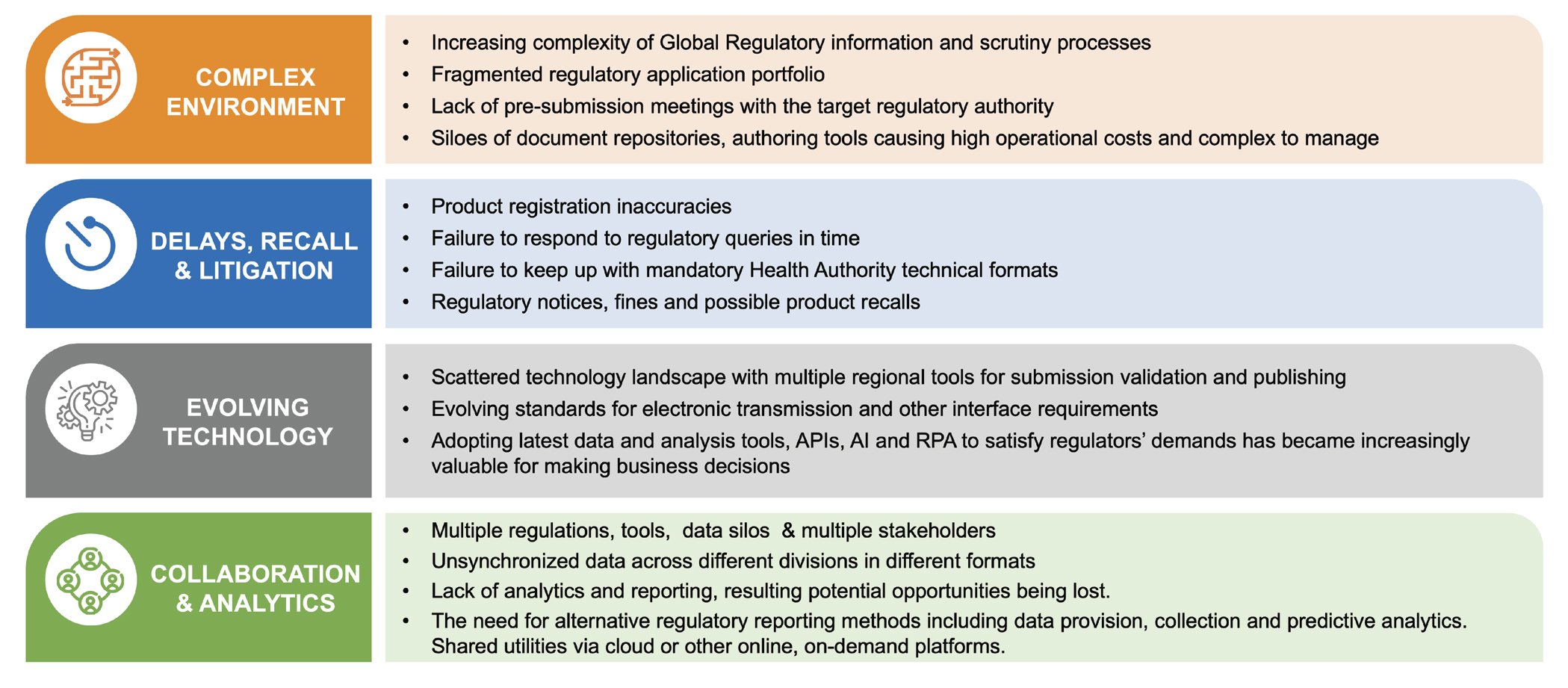
Regulatory compliance is one of the most crucial aspect in life sciences and lately, there has been a set of evolving global compliance standards that organizations are obliged to meet. Compliance has become more demanding in terms of effort and cost. The complexities of regulations leading to a global compliance anxiety are multifaceted; some of them are as below:
New regulations & Legal Liability: XEVMPD, IDMP, RPS are some new & evolving regulations. Drug makers exaggerating their benefits and urging doctors to over-prescribe for the sake of increasing revenue has been a trigger of some new regulations.
Increasing scrutiny & Patent Cliffs: Product recalls and humongous llitigations sometimes running into tens of billions of dollars are a major challenge. Another toll on the industry is the patent cliff, which is expected to risk $50 billion worth of prescription drug sales worldwide in 2022.
Keeping Up with Technology: 3D printers could emerge as top competitors for manufacturers; the first printed drug was approved by the FDA in 2015
Increasing costs & Product Liability: $850 m average cost for a new drug to market 30% of which is on regulatory contents.
Increasing operational complexities: 35% non-compliance on regulatory standards and guidelines. There is also increased competition from Generic Drugs.
Emerging market growth & Generic Drugs: Emerging markets throw in their own complexities concerning regulations. In 2017, the FDA began a push to get lower-cost generic drugs out to market faster.
Challenges
Solution
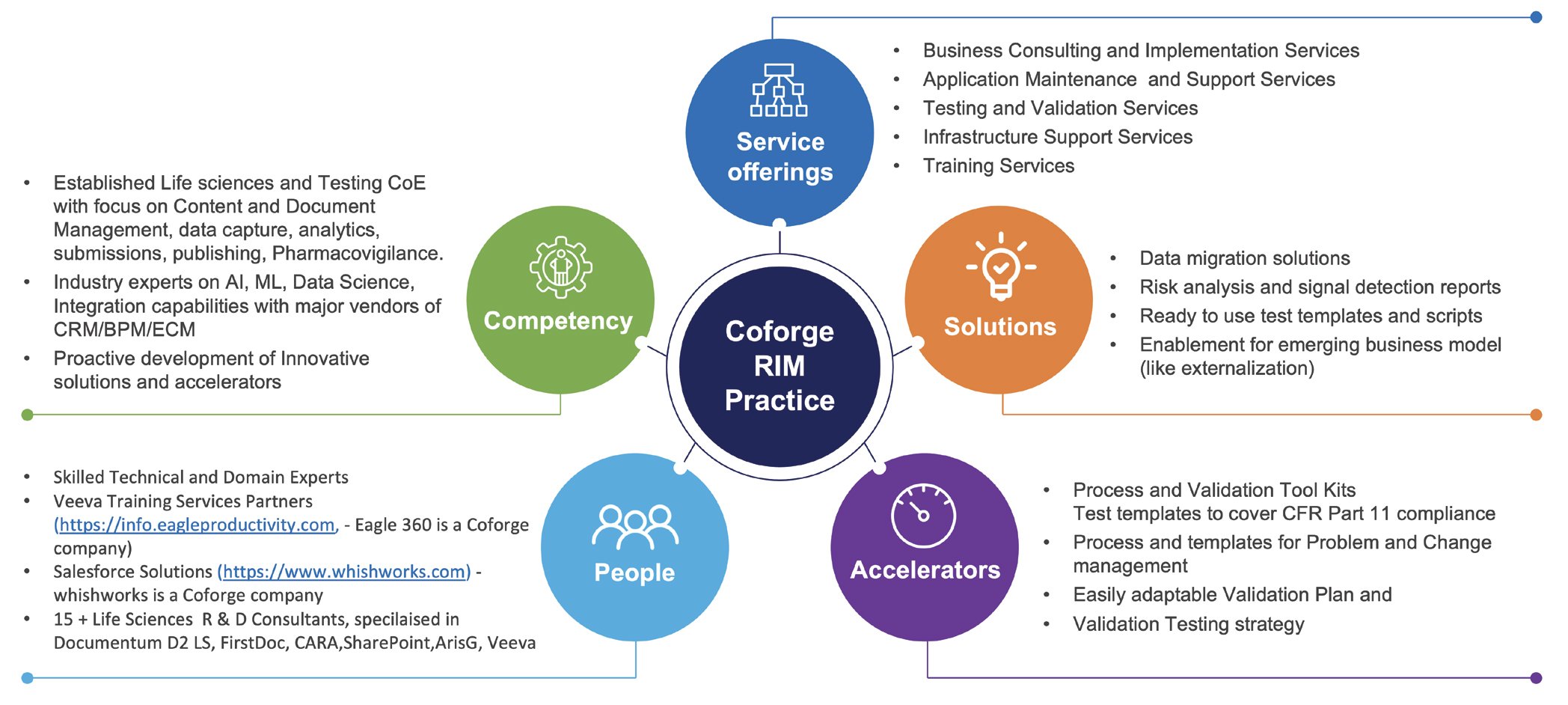
Regulatory Information Management: Coforge's RIM practice is designed to deliver services that compliments the core product for a holistic coverage. Our resources are skilled and ready to be deployed, saving about 25% in ramp up time. There would be drastic reduction in cost on product training clubbed with quick and efficient transition. We also have ready to use solutions demanding minimum changes for a time and cost effective deployment. Our testing and validation accelerators can reduce the overall preparation and execution effort by about 25%.
Regulatory Submission Platform: Coforge offers a best in class solution designed exclusively for the pharmaceutical industry for smooth working of R&D, manufacturing, and quality operations. The end-to-end prebuilt, preconfigured and hosted solution leverages leading COTS products as standard interfaces with single-sing-on and unified user interface. The solution has a secured framework to address Pharma security and privacy. Our unique service delivery model enables pay per use on volume of usage and resource consumption with minimal initial investment. We also bring in the comfort of a single touch point for higher accountability. Coforge's platform is bolstered on strategic partnerships with leading product vendors to provide best in class solutions. We bring in flexibility to meet usage expansions and performance standards with ISO27001 certified cloud partners such as Azure, AWS, Google, and Cloud Foundry.