The pharmaceutical supply chain is a complex, multi-stakeholder ecosystem, often hindered by fragmented systems and limited visibility. Compounding the challenge is the ease with which drug packaging, essentially artwork, can be replicated, enabling the infiltration of counterfeit or spurious drugs into the supply chain.
One leading pharmaceutical company reported that up to 0.5% of its annual production for a blockbuster drug was identified as counterfeit in the market.
This isn’t just a financial risk. Counterfeit drugs compromise patient safety, disrupt therapeutic outcomes, and erode brand trust. As regulators and governments tighten control, pharma enterprises must now treat Track & Trace as both a compliance requirement and a strategic differentiator.
To address these vulnerabilities, the U.S. enacted the Drug Supply Chain Security Act (DSCSA) in 2013, mandating full interoperability across the pharmaceutical supply chain by 2023. This regulation requires end-to-end traceability down to the individual unit level.
This blog explores the implications of the DSCSA’s ‘Track & Trace’ mandate and outlines a digital solution framework designed to fulfill these interoperability and traceability requirements.
The Pharma Supply Chain: Challenges & Fragmentation
The pharmaceutical supply chain spans a vast network of stakeholders, including manufacturers, clearing and forwarding agents (CFAs), distributors, retail pharmacies, and ultimately, the end consumer.
However, this supply chain is often fragmented and lacks interoperability, creating significant vulnerabilities. These gaps make it easier for counterfeit drugs to infiltrate the system, leading to serious consequences such as revenue loss for pharmaceutical companies, costly drug recalls, and, most critically, adverse patient outcomes.
Many organizations still rely on paper-based systems or isolated digital tools, limiting real-time traceability. Without a unified view of T3 data (Transaction History, Transaction Information, Transaction Statement), companies struggle to comply with DSCSA mandates.
Regulatory Push: DSCSA’s Impact on Stakeholders
In the US, the Drug Supply Chain Security Act was made into a law in 2013 to address these shortcomings; it mandates the pharma supply chain to be completely interoperable by 2023, allowing traceability down to an individual unit.
With this regulation, all the key stakeholders such as Manufacturer, Repackager, Wholesale Distributor, 3PL provider, and Dispenser would be part of an interoperable ecosystem with a focus on sharing Transaction Information, Transaction Statement, and Transaction History referred to as T3.
Beyond DSCSA, global regulatory movements like the EU Falsified Medicines Directive (FMD) and WHO’s GSMS framework are pushing for harmonized, digital-first traceability systems. These regulatory shifts are accelerating the move towards cloud-based and blockchain-enabled Track & Trace platforms.
Coforge’s Digital Framework for End-to-End Traceability
Based on our deep industry experience, Coforge provides guidelines for the solutions that can enable supply chain visibility, ensure authenticity checks, be scalable, and improve customer brand experience. The guidelines for a comprehensive Track & Trace solution are:
Serialization – Solution should be able to fulfill serialization requirements across all pharma drugs at a unit level, primary packaging level, secondary packaging level, and pallet. These solutions should be compatible with high-speed artwork printers/ their middleware or propose changes to the production line with minimal disruption.
Authenticity – Solution should be able to drive ‘Authenticity check’ to distinguish a fake drug from a genuine one. Multiple technologies like numeric hash, Blockchain, and invisible crypto signatures are available. Technologies that deter imitability should be preferred. Blockchain and invisible crypto signatures are the most difficult to track of the available technologies.
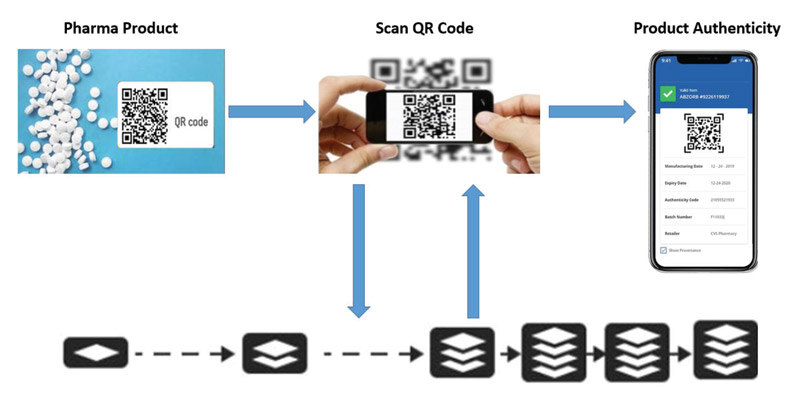
Encoding - QR Code/ Bar Code – The Solution should be able to generate QR codes/ barcodes in real time for all units, including primary & secondary packaging, to be applied on artwork. This system should be integrated with the Serialization software /middleware.
Patient App/ Website – The customer app/portal should be available for a seamless digital experience, enabling easy validation of a drug with the manufacturer and empowering the stakeholders with complete visibility of the supply chain in order to comply with DSCSA regulations. This app/ portal should open after scanning the QR Code/barcode.
Dynamic branding Content – Branding / Labelling / Therapeutic content should also be made available to the customer. This page/site can contain details like usage, safety instructions, storage instructions, videos, patient support programs, etc., which provide more engaging content for the patient.
Provisioning – For all active supply chain partners like CFAs, distributors, retailers, pharmacies, digital solutions like app/ web portals should be provided to record the delivery acceptance. This helps to trace the journey of the drug till the last mile.
Reporting – Functionality of reporting spurious /counterfeit goods should be made available. This can happen through the patient app/portal. Typically, the image of the packaging, email, and geography should be captured while recording the complaint. For non-users, like HCPs and caregivers, who might not scan the QR code, but still have to report counterfeit, additional avenues like web portals and call centers should be made available.
Dashboards – Dashboards and reporting facilities should be part of this solution. Most common dashboards are Geographic distribution/ insights on the incidents raised on the basis of region, category, and product. Supply Chain Visibility Dashboard to enable Track & Trace of drugs.
Conclusion: Coforge as a Strategic Partner
Coforge brings a blend of technology expertise, domain depth, and global experience in delivering real-world Track & Trace solutions.
- Trusted integrations with partners like SAP, TraceLink, Salesforce, ACG, and Traceready
- Customizable, hybrid deployment models—on-prem, cloud, or federated
- Deep GxP knowledge ensures compliance, audit-readiness, and scalability
With our modular Quasar platform and AI/ML capabilities, we help clients go beyond compliance—unlocking real-time intelligence, risk prevention, and smarter pharma supply chains.
Connect with us to explore how Coforge can help you design a resilient, compliant, and intelligent Track & Trace solution across your global supply chain.
Visit Coforge Healthcare & Life Sciences to learn more.



